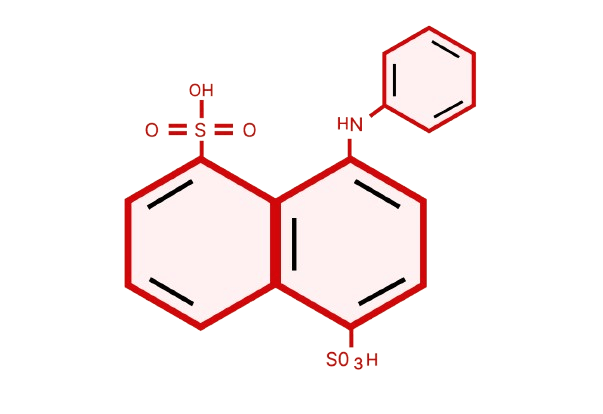
Laurent's Acid | 84-89-9 MSDS (Material Safety Data Sheet) or SDS, COA and Specs
Laurent's Acid | 84-89-9
| CAS No. | 84-89-9 |
| Formula | C10H9NO3S |
| EINECS | 201-571-0 |
| Molecular Weight | 223.25 |
| Appearance | White or pale pink needle-like crystal |
| Density | 1.502 g/cm3 |
Introduction and Exploring Laurent's Acid
The industry of color, whether for leather, textiles, or inks, relies heavily on high-performance dye intermediates for uniformity, vibrancy, and durability. Of these, Laurent's acid is a multi-purpose compound in many chemical dyes.
A prominent Laurent's Acid producer in India, Apurva Chemicals offers CAS 85-47-2 grades of products that exceed the highest purity, uniformity, and compliance with regulatory requirements. This blog examines the value of Laurent's Acid, its application, and why India is the preferred destination for buyers worldwide.
What is Laurent's Acid?
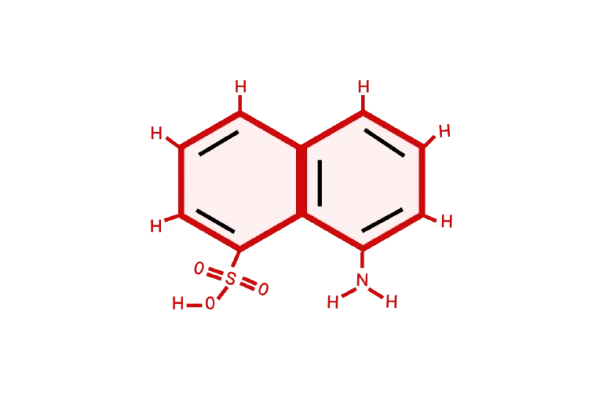
Laurent's Acid, chemically known as 1-Amino-8-naphthol-3,6-disulfonic acid, is a key intermediate in synthesizing various azo dyes, reactive dyes, and pigments. It is also called NYS-1,5-Laurent’s Acid. The organic compound is admired for its sturdiness, solubility, and compatibility with multiple chromophores and coupling agents.
Chemical Properties & Structure
- CAS Number: 84-89-9
- Molecular Formula: C10H9NO7S2
- Appearance: Off-white to beige crystalline powder
- Solubility It is soluble in water and particularly in alkaline circumstances.
- Functional Groups The amino group is a hydroxyl group and two sulfonic acid groups.
Its properties allow it to function as a co-coupling element and an intermediary when it comes to the development of colors.
Applications in Dyes and Pigments
Laurent's Acid has a vital role in the process of synthesis
- Azo dyes to dye leather and textiles
- reactive dyes are suitable for cotton as well as blended fabrics
- Acid dyes for silk and wool
- Pigments to be used in Inks, plastics and coatings
- Intermediate to optical brighteners as well as pharmaceuticals.
Because of its dual sulfuric acid groups and hydroxyl arrangement, it has high reactivity and binding strength, particularly in high-temperature processes for dyeing.Laurent's Acid, a dye intermediate, works with Peri Acid to create vivid colorants.
Benefits of High-Purity Laurent's Acid
Selecting a Laurent's high-purity Acid has distinct benefits:
- Better the yield of dye and the brightness
- More stable in solutions containing aqueous solutions
- Improved water and light-weight of finished products
- lower impurity levels and sodium levels, assuring the environmental quality
- More formulation flexibility in specialty dyes
This is particularly important for export-grade textiles and highly valuable dyes used for technical purposes.
Industrial Manufacturing & Specifications
We are Apurva Chemicals; we manufacture Laurent's Acid inside an industrial setting that is controlled and adheres to the strictest specifications for quality.
- Purity: >=98%
- Moisture content: <2%
- Free acid content: Negligible
- Metals heavy: Compliant to international standards for safety
We also provide specific specifications that are custom-based on the formulations of our clients as well as industry standards.
India's Role in Global Laurent's Acid Supply
India is the dominant player in the world market for dye intermediates, renowned for:
- Well-established chemical infrastructure
- The raw material of economic value access
- strict conformity to international trade standards
As a reputable Laurent's Acid production company in India, We help international buyers cut costs on sourcing without sacrificing quality.
Why Choose Apurva Chemicals?
As a pioneer in dye intermediate manufacturing, Apurva Chemicals offers:
- State-of-the-art production facilities
- Labs in-house to help with testing and customizing the product
- REACH-compliant proce...